Gas Chromatography Explained: What It Is and How It Works
If you study pollutants in the air and water or control food or beverage quality, you’re probably familiar with a GC detector and know quite a bit about Gas Chromatography (GC).
This article is for the rest of us. Those who may be a little familiar with the term but could use a really good definition of what GC is and how it works.
Botanist Mikhail Tswett has been credited for creating the first chromatogram in 1900 to study plant pigments, including chlorophyll and carotenes. Over time, its use has spread to many other applications for separating complex mixtures.
Currently, gas chromatography is widely used for product quality control, analytical research, and safety testing relevant to key aspects of everyday life—from car production to chemical refining and pharmaceutical industrial use, to food and beverage sampling.
Gas Chromatography Definition and Analogy
So, what is GC anyway? It is an analytical technique used to separate the chemical components of a sample mixture and then detect them to determine their presence or absence. It is also used to figure out how much is present in the sample. Organic molecules and permanent gases are the most common chemicals analyzed by GC. It can analyze compounds in the boiling range from nC1 to nC100 as is the case with crude oil; however, the most applications are in boiling point range from nC3 to nC44.
The word chromatography comes from the Greek root words chroma and graph and translates to “color writing.” GC is a technique that separates a mixture of chemicals by letting them move slowly past another substance, typically a liquid or solid. In essence, we have a gas moving over the surface of something else in another state of matter (a liquid or solid) that stays where it is. The moving substance is called the mobile phase and the substance that stays put is the stationary phase.
GC uses an inert or unreactive carrier gas as the mobile phase, and the stationary phase is generally a thin layer of liquid. As the mobile phase moves, it separates the mixture into its individual components in the stationary phase. We can then identify them one by one.
5 Simple Steps For GC, 1 Giant Leap for Chemists Everywhere
A Gas Chromatograph is comprised of a heated inlet port, an oven, an analytical column, and a detector. Let’s walk through the process:
Step 1: Sample Prep
Samples are generally dissolved or diluted in a solvent and then injected onto the inlet port. Some samples such as essential oils are not diluted. Other methods of sample preparation, include sample introduction techniques such as thermal desorption and headspace. These are easy techniques for there is typically no or minimal sample preparation.
Step 2: Vaporization
This is as simple as it sounds. The liquid sample is vaporized in the hot inlet and becomes a gas.
Step 3: Separation
Here an inert gas such as helium carries the sample through the column. Different substances in the sample interact differently with the column’s stationary phase, depending on their chemistry. This causes them to travel through the column at different speeds, thus separating them.
Step 4: Detection
The separated compounds then leave the column one after the other and enter a detector, such as a mass spectrometer (MS). The use of a MS detector is helpful. Because of the huge amount of organic compounds, at times, compounds may elute at the same time. A MS helps identify what the compound is and separates them by mass. The time taken for a compound to travel through the column is called its retention time.
Step 5: Chromatogram
The GC produces a graph called a chromatogram, which shows peaks: the size of a peak indicates the amount of each component reaching the detector. The number of peaks shows different compounds present in the sample. The position of each peak shows the retention time for each compound.
Figure 1: How does GC work.
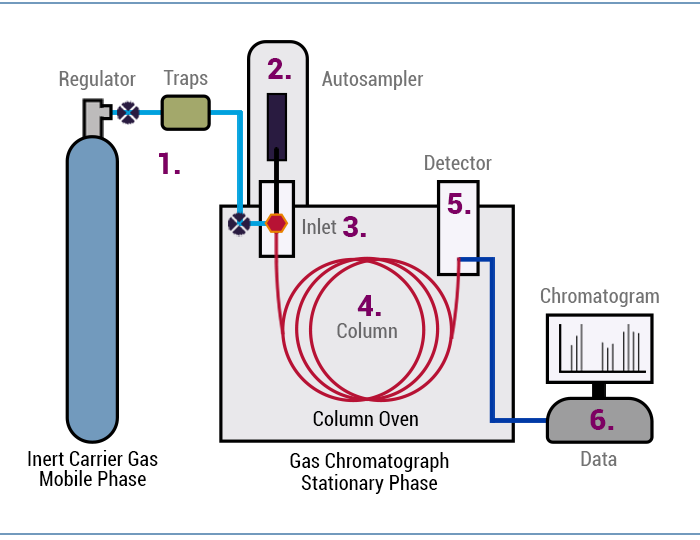
As illustrated above, the carrier gas transports the sample molecules through the GC system.
How Does Gas Chromatography (GC) Work?
- The sample is first introduced into the GC, with a syringe most commonly from a liquid autosampler (Figure 1 (2))
- The sample is injected into the GC inlet (Figure 1 (3)) through a septum which enables the injection of the sample mixture without losing the mobile phase. Connected to the inlet is the analytical column (Figure 1 (4)), a long (10 – 150 m), narrow (0.1 – 0.53 mm internal diameter) fused silica or metal tube which contains the stationary phase coated on the inside walls.
- The analytical column is held in the column oven which is heated during the analysis to elute the compounds of different boiling points.
- The outlet of the column is inserted into the detector (Figure 1 (5)) which responds to the chemical components eluting from the column to produce a signal.
- The signal is recorded by the acquisition software on a computer to produce a chromatogram. (Figure 1 (6), (Figure 2) If required, the software can quantitate or produce an amount of that compound referencing a known amount of that compound.
When You Think GC—Think Separation and Detection
Simply said, GC is used to separate the chemical components of a sample and then detect them to determine their presence or absence. It can also be used to know how much of something is present in the sample.
Discover PerkinElmer Gas Chromatography Portfolio and how it can help your lab with GC analysis.
Footnote
- https://www.technologynetworks.com/analysis/articles/gas-chromatography-how-a-gas-chromatography-machine-works-how-to-read-a-chromatograph-and-gcxgc-335168
- Derivatization is a technique which converts a compound into a product (the reaction’s derivate) of similar structure called a derivative. Polar N-H and O-H groups on which give hydrogen bonding may be converted to relatively nonpolar groups on a relatively nonvolatile compound. The resultant product may be less polar, thus more volatile, allowing CG analysis. Source: https://web.archive.org/web/2016304002519/https://www.chromspec.com/pdf/gc_derivatization_methods.pdf
Other sources used to write this blog: https://pubmed.ncbi.nlm.nih.gov/8075776, https://elscience.co.uk/our-lab/gcms-work/, https://www.explainthatstuff.com/chromatography.html

