How High-Performance Liquid Chromatography (HPLC) Works
High Performance Liquid Chromatography (HPLC) is an analytical technique that is ubiquitous in labs across the globe. This technique is commonly used in labs for the purpose of separating, identifying, and quantifying components in a mixture. This post continues the series of Chromatography Explained and will focus on introducing HPLC and how HPLC instrumentation works.
Scientists A. J. P. Martin and R. L. Millington Synge were the first to develop a chromatographic approach using two liquid phases instead of one, traditionally used in gas chromatography. The two liquid phases enable the separation of compounds with different partition coefficients. This is considered the beginning of the development of high-pressure liquid chromatography and earned the two scientists the Nobel Prize in Chemistry in 1952.
High-Performance Liquid Chromatography (HPLC) Definition and Introduction
High-pressure liquid chromatography (HPLC), also known as high-performance liquid chromatography, is a more advanced type of liquid chromatography (LC) that became prevalent about 20 years after Martin and Synge won the Nobel Prize. HPLC is especially useful for low or non-volatile organic compounds, which cannot be analyzed with gas chromatography.
The technique relies on a mobile phase and a stationary phase to separate components within a mixture. A high-pressure pump delivers the mobile phase through the system. Compounds with a higher affinity for the mobile phase will migrate through the column more rapidly and interact less with the stationary phase.
Once separated, a detector measures the concentration of the analytes and converts them into electrical signals; the concentration of each component is proportional to the amount that was eluted from the column. The time taken between injection and detection – known as the retention time – is specific for a given set of chromatographic conditions and may be compared with a standard for identification.2,3
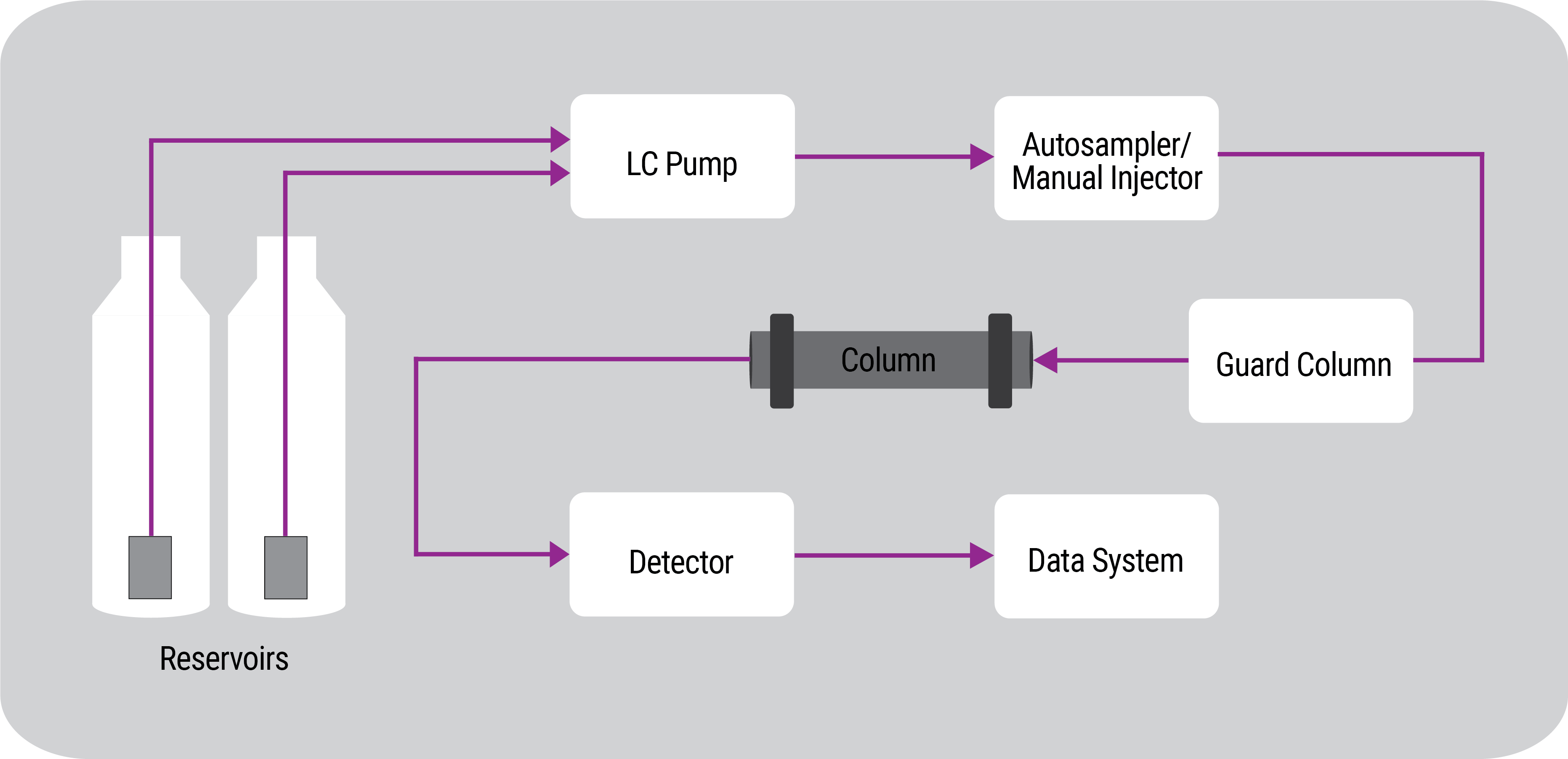
HPLC in Four Steps
- The sample is first dissolved in a liquid or the mobile phase.
- This solution is then injected by means of a manual injector or an autosampler into a continuous flow of mobile phase, being delivered by a pump, and carried onto the LC column which contains a stationary phase.
- The various components of the sample travel through the column at different speeds due to their interactions between the mobile and stationary phases, resulting in the components separating from one another. The different travel times are referred to as the components’ retention time.
- When components emerge from the column, they are carried to a detector where a physical property of the compounds is measured, such as absorption of light for UV detection.
It’s important to note that there are many different detectors available. Some of the most common detectors are ultraviolet/visible (UV/Vis), photodiode array (PDA), fluorescence (FL), and refractive index (RI). Each response plotted time, resulting in a chromatogram.
Learn how PerkinElmer Liquid Chromatography Portfolio help your lab with HPLC analysis.
Footnotes:
1.https: /www.chromatographytoday.com/news/gc-mdgc/32/breaking-news/who-is-mikhail-tsvetnbsp/32304
2.Ibrahim D, Ghanem A. Sub-2 μm silica particles in chiral separation. In: Pagnola MR, Vivero JU, Marrugo AG, eds. New Uses of Micro and Nanomaterials. InTech; 2018.
3.Dong MW, Wysocki J. Ultraviolet detectors: Perspectives, principles, and practices. Chromatographyonline.com. https://www. chromatographyonline.com/view/ultraviolet-detectors-perspectives-principles-and-practices
Additional Reference Material:
The Basics of HPLC/UHPLC and LC Columns Training Guide, https://www.perkinelmer.com/libraries/gde-the-basics-of-hplc-uhplc-and-lc-columns-training-guide