Overcoming Challenges in CHO Host Cell Protein Detection and Quantification
Over two-thirds of recombinant biopharmaceuticals, including more than 85% of monoclonal antibody drugs, are manufactured using Chinese Hamster Ovary (CHO) cell lines. Residual Host Cell Protein (HCP) impurities derived from these cells can compromise the stability, efficacy and safety of the therapeutic product.
Regulatory authorities such as the FDA therefore require manufacturers to monitor and demonstrate HCP clearance, and to report the amount of HCP impurities in the finished product. Although HCP detection methods are well-established, many labs struggle with assay development and qualification. In particular, there are a number of factors to consider when selecting and validating an HCP ELISA, which is the workhorse assay for CHO-HCP detection and monitoring.
Below we address some of the most common questions and main challenges of CHO-HCP impurity testing.
What are host cell proteins (HCPs)?
HCPs are proteins endogenous to the host expression system or cell substrate used to produce a therapeutic drug product. Many HCPs are actively secreted by host cells into the surrounding culture medium. Others may be released during the course of cell death or upon cell lysis—for example, during routine cell passaging or harvest procedures.
Most HCPs are very low in abundance relative to the therapeutic product, and many are fairly easy to remove by downstream purification steps such as affinity chromatography and size exclusion. However, some HCPs have the potential to co-purify with therapeutic products, making their removal more difficult and potentially compromising product quality or clinical safety.
What are the risks of HCP impurities and how are they regulated?
Immunogenicity of HCPs is one of the biggest concerns for drug developers and regulators because of the implications for patient safety. The human immune system is exquisitely sensitive to detect and mount a defensive response to any foreign protein it encounters. This means that under the right circumstances virtually any HCP from a “non-self” host, such as a CHO cell line, could trigger a life-threatening immune response or persistent sensitivity in patients.
Biological activity of HCPs is another major concern. Many co-purifying HCPs are enzymes that can degrade the therapeutic product or formulation excipients—even when present at extremely low levels. Over time, this activity can reduce drug stability, efficacy or potency in the final formulation. Some HCPs have biological activities that can mediate on-target or off-target toxicological effects in the patients. These include various cytokines and chemokines, hormones, regulatory molecules and enzymes.1
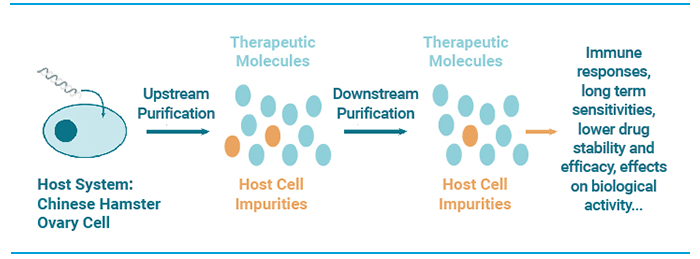
Figure 1. What are CHO HCPs?
Why is HCP detection and assay development so challenging?
Regulatory agencies class HCPs as a critical quality attribute (CQA) that must be monitored and reported. The central challenge in HCP detection and monitoring is that HCP populations are highly heterogeneous and variable. Samples collected at various points during bioprocessing may contain hundreds or even thousands of different protein species with vastly differing physicochemical properties. With each successive step of bioprocessing, the HCP profile changes. Technology choices and variations in processing conditions can also significantly alter the total amount and relative levels of individual HCP species found at each stage of the process.
Establishing a single assay with broad enough coverage to detect the majority of HCP species in any given sample is therefore a tall order. This is further complicated by the need for high sensitivity of detection (as low as a few parts per million) and a wide enough dynamic range to be able to accurately and reliably monitor changing levels of low-abundance HCPs against a backdrop of high levels of therapeutic protein.
Which assay type is most appropriate: generic or process-specific?
HCP testing plays a crucial role in developing, validating and controlling the biopharmaceutical manufacturing process. It is instrumental in demonstrating HCP clearance from production lots that will be used for process validation and preclinical toxicology studies, and is required for regulatory release of material prior to clinical studies and commercial use.
Enzyme-linked immunoassay (ELISA), particularly the sandwich ELISA, has become the methodology of choice to quantify total HCP in bioprocess development and manufacturing. At their core, HCP ELISAs rely on high-quality polyclonal antibody reagents to provide broad coverage, sensitivity and specificity for heterogeneous HCP populations.
Depending on the antigen use for polyclonal antibody generation, HCP-ELISAs can be generic or process-specific. The question of which assay type to use when is not always clear-cut. Generic assays provide maximum flexibility during the early stages of pre-clinical and clinical development, when HCP populations are highly variable. This is because the anti-HCP antibodies are designed to recognize a broad spectrum of HCPs representative of a variety of different upstream process procedures and common host expression strains.
Generic HCP assays become less relevant or appropriate during the latter stages of clinical development, especially from the start of phase III clinical trials onwards, when drug substance must be produced to the same specifications as the commercial product.
At this point, a more tailored, process-specific, HCP assay is usually required to adequately monitor the unique HCP population associated with the approved bioprocess. However, generic HCP assays can sometimes be used for late-phase development, provided there is sufficient data to demonstrate that the assay meets the required performance and coverage criteria.
Performance Criteria for Generic HCP Immunoassays
To qualify for use in bioprocess development and manufacturing, HCP immunoassays must be well-characterized and satisfy stringent performance criteria. From a regulatory standpoint, some of the most important criteria to consider during assay validation include:
Coverage
Demonstrating sufficient coverage to detect the broad spectrum of HCP species expected in various bioprocess samples is a key validation requirement. Coverage capability of anti-HCP polyclonal reagents is typically assessed using two-dimensional differential in blot electrophoresis (2D-DIBE) or HCP affinity chromatography.
Regulatory guidelines set no minimum coverage specification, instead recommending that coverage be as broad as possible within the limits of practicality. For generic commercial CHO-HCP immunoassay kits, it is reasonable to expect at least 50% coverage (as assessed by 2D-DIBE) for the CHO cell strains most commonly used in commercial biopharmaceutical manufacturing (e.g., CHO-S, CHO-K1, CHO-DG44), although coverage as high 80% is considered achievable with current technologies.
Sensitivity
Since even small amounts of certain HCPs can be immunogenic or affect product stability, high assay sensitivity is essential. The ability to reliably detect HCP concentrations as low as a few nanograms per mL is highly desirable for risk mitigation. Assay sensitivity is typically expressed in terms of the lower limit of detection (LOD). The limit of quantitation (LOQ) is also a useful criterion, indicating the lowest concentration that can be measured with acceptable precision and accuracy.
Matrix effects
When testing complex biological samples such as crude culture supernatant, it is important to demonstrate that there are no substances in the sample that could interfere with (depress or enhance) the assay signal to skew the results. The potential for these matrix effects is assessed by running the assay on mock samples that have been spiked with a known amount of HCP analyte. The analyte concentration measured by the assay is then expressed as a percentage of the expected concentration. Antigen recovery values in the range of 80 -120% are usually considered acceptable.
Linearity and dynamic range
Good dilutional linearity across a broad range of protein concentrations is crucial to ensure acceptable accuracy and precision of HCP measurements. Multi-point linearity-of-dilution and spike-and-recovery assessments are typically performed across an expected concentration range from 0-200 ng/mL.
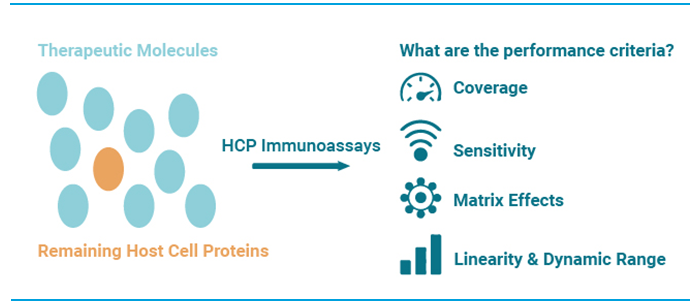
Figure 2. Performance criteria for generic HCP immunoassays
Improving and Accelerating CHO-HCP Testing
recent years, significant progress has been made in HCP immunoassay technologies. In particular, a number of advances have been made to improve the sensitivity of immunoassay technologies, remove the need for wash steps, and streamline assay workflows for easier automation and increased throughput.
The sandwich ELISA (Figure 1) is one the most widely used immunoassay formats for HCP quantification. By sandwiching antigen between two HCP-specific antibodies, this format generally achieves higher specificity and sensitivity than conventional direct ELISAs. In addition, the antigen does not need to be purified prior to measurement, making this type of assay a good choice when analyzing complex HCP samples, such as cell culture supernatant or crude cell extracts.
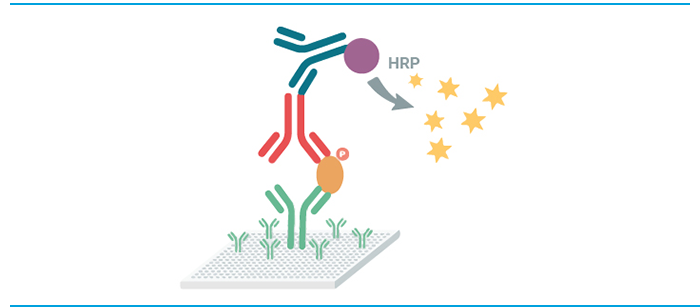
Figure 3. Sandwich ELISA principle
One concern with ELISA assays in HCP testing is the need for multiple reagent additions and wash steps that contribute to assay variability and complicate automation. HTRF assays (Figure 2) offer a no-wash alternative that combines standard FRET technology with time-resolved fluorescence measurements. The technology enables a simplified “mix-and-read” assay format that is more robust, sensitive, and easier to automate than conventional ELISAs. These benefits make HTRF a popular choice for high-throughput applications.
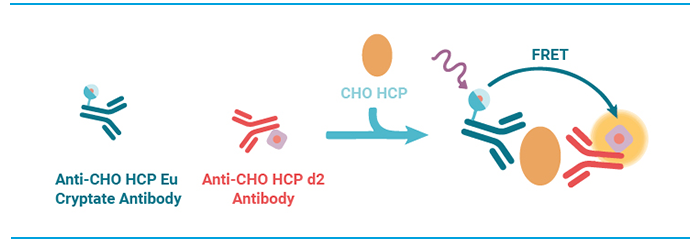
Figure 4. HTRF assay principle.
Another assay approach that overcomes many limitations of standard immunoassays is AlphaLISA® (Figure 3). Like HTRF, AlphaLISA is a bead-based proximity technology which eliminates the need for wash steps. What sets this approach apart from other immunoassay approaches is the use of luminescent oxygen-channeling chemistry. This provides superior signal amplification compared to conventional enzymatic amplification. Background interference is also greatly reduced because the wavelength of emitted light is shorter than the excitation wavelength.
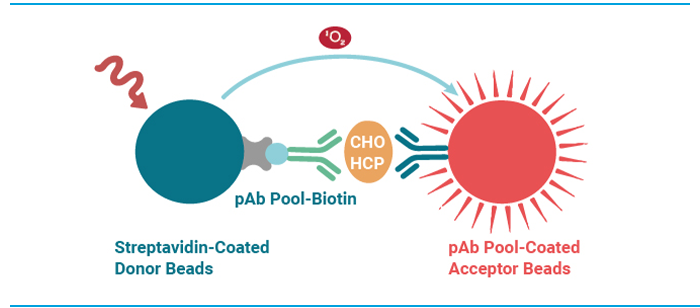
Figure 5. AlphaLISA assay principle.
Get your Guide and Application Note
If you want to learn more about the latest tools and strategies for HCP detection, download our comprehensive new guide, “Detecting CHO Host Cell Proteins in Biopharmaceutical Development and Manufacturing.”
For a detailed demonstration of how HTRF and AlphaLISA CHO-HCP assay kits can increase both effectiveness and efficiency when incorporated into your existing workflows, read our related Application Note, “Streamline the CHO HCP Impurity Quantification Workflow with New High Throughput, No-wash HTRF® and AlphaLISA® CHO Host Cell Protein Kits.”
Note:
- https://www.biophorum.com/host%20cell%20proteins/ offers the latest information on this subject, including a comprehensive list of potentially problematic and high-risk CHO HCPs.