FTIR and the Science of Sandals
Summer means sun, sandals, and always working in the background… science. Let’s take a break from our summer read, and read about how Fourier transform infrared spectroscopy (FTIR) can assure that footwear designers keep you comfortable.
Traditionally, natural materials such as wood, cork, and leather were used to construct sandals, shoes and boots, but synthetic polymer-based materials have expanded the realm of possibilities significantly. These materials can offer increased durability over natural materials and in many cases, the mechanical properties can be fine-tuned by adjusting the chemistry or processing of the material. This allows the material to conform quickly to the wearer’s foot, instantly delivering comfort and absorbing shock. Just as important is the ability of the material to return to its original shape following compression.
The figure below shows two common synthetic materials used in footwear – ethylene vinyl acetate (EVA, upper, in black), and polyurethane (PU, lower, in red). Ethylene vinyl acetate is readily identified by the strong bands of the C-H stretch (2916, 2849 cm-1), ester carbonyl band (1739 cm-1), methyl and methylene deformation (1466, 1372 cm-1), and the C-O stretch bands (1239, 1019 cm-1), as well as a methylene chain rock band (720 cm-1).
Other bands are present in the spectrum are those of fillers and pigments such as calcium carbonate (1430 and 876 cm-1), a silica or silicate (1009 cm-1), and titanium dioxide (broad 700-500 cm-1). The mechanical properties of this material can vary with the vinyl acetate content, with higher vinyl acetate content causing the material to become rubbery and flexible. Such compositional differences are reflected by changes in the relative peak intensities in the infrared spectrum.
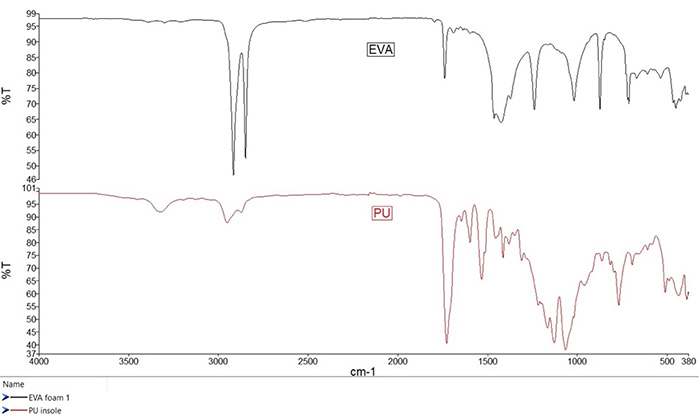
FTIR spectroscopy of ethylene vinyl acetate (EVA, upper, in black), and polyurethane (PU, lower, in red)
Polyurethanes are made from a wide range of monomers, and so represent a broad category of materials, rather than a specific polymer composition. The material is generally made from two monomers – an isocyanate and a polyol, which are the functional groups where the urethane linkages form during the polymerization process. As this is the defining characteristic of the material, one can expect to see a single, broad, “v”-shaped peak near 3330 cm-1, which is the N-H stretch.
This very same bond can give rise to a nearby, weak overtone of the N-H deformation, but assignment of this band may be confounded when examining urethanes with aromatic monomers, as the aromatic C-H bond stretch will also appear nearby. The urethane group also contains a carbonyl, which gives rise to the band at 1728 cm-1. Deformation of the N-H bond causes the peak observed near 1533 cm-1, and the C-O bond gives rise to the intense peak near 1200 cm-1. Other bands across the fingerprint region are exquisitely sensitive and unique to the structure of the starting monomers, which is helpful where suitable reference spectra or libraries are available.
These materials can be rapidly identified using the Search or Compare algorithms in the Spectrum IR software, eliminating the need for manual interpretation of spectra. Additionally, PerkinElmer’s proprietary Atmospheric Vapor Compensation (AVC) algorithm automatically subtracts spectral features of water vapor and carbon dioxide, which would otherwise adversely affect library search results and hamper visual inspection.

