Mass Spectrometry Explained: How LC/MS/MS Works
The need for faster and more efficient analysis of larger and more complex samples has driven the development of advanced instruments in the lab, leading to the creation of improved instruments capable of handling increasing demands for productivity and accuracy. For the identification and quantification of compounds, liquid chromatography (LC) coupled with mass spectrometry (MS) has become the technique of choice due to their separation capabilities and resolving power.
Triple quadrupole mass spectrometers (LC/MS/MS) are established in labs dealing with challenging matrices, as they offer very low detection limits with specificity and selectivity. These factors positively impact the efficiency and cost-effectiveness of laboratory operations.
Additionally, LC/MS/MS solutions have been improved to add automation and reduce the complexity of the analytical workflow while increasing lab uptime and reducing maintenance needs.
This post will provide background on LC/MS/MS and explain how it works.
LC/MS/MS Definition and An Introduction
J.D. Morrison of La Trobe University, Australia, and Prof. Christie G. Enke and his then-graduate student Richard Yost, pioneered the linear arrangement of three quadrupoles in the first triple-mass spectrometer.1 Enke and Yost built the first commercial triple-quadrupole mass spectrometer in 1978 at Michigan State University.2 During the 1980s, scientists found that the triple-quadrupole mass spectrometer could help them study organic ions and molecules, thus expanding its capabilities as a tandem MS/MS technique.3
LC/MS/MS analytical workflow starts with a liquid chromatography instrument (HPLC) separating and concentrating sample components before reaching the MS. In the mass spectrometer, quadrupoles filter ions by applying an oscillating radio frequency combined with a direct electric field, which leads to the separation of ions based on their mass-to-charge ratios (m/z). Within the triple quadrupole configuration, the first quadrupole isolates analyte molecular ions, the second quadrupole fragments select molecular ions, and the third quadrupole selectively isolates the ion fragments for measurement via a detector.
Though an LC/MS/MS system is known as a triple quadrupole instrument, it is technically a tandem quadrupole mass analyzer. The first and third quadrupoles are mass analyzers, while the second quadrupole functions as a collision cell. This method yields greater structural information, enhanced selectivity, and sensitivity and ensures a robust and clean signal.
Analysts looking for a specific mass from a sample benefit from mass spectrometers with single quadrupoles as they enable the selection of a specific mass from a relatively clean sample matrix. However, they require triple quadrupole instruments to differentiate between molecules with the same mass or discern between analytes in a complicated mixture or a complex matrix.
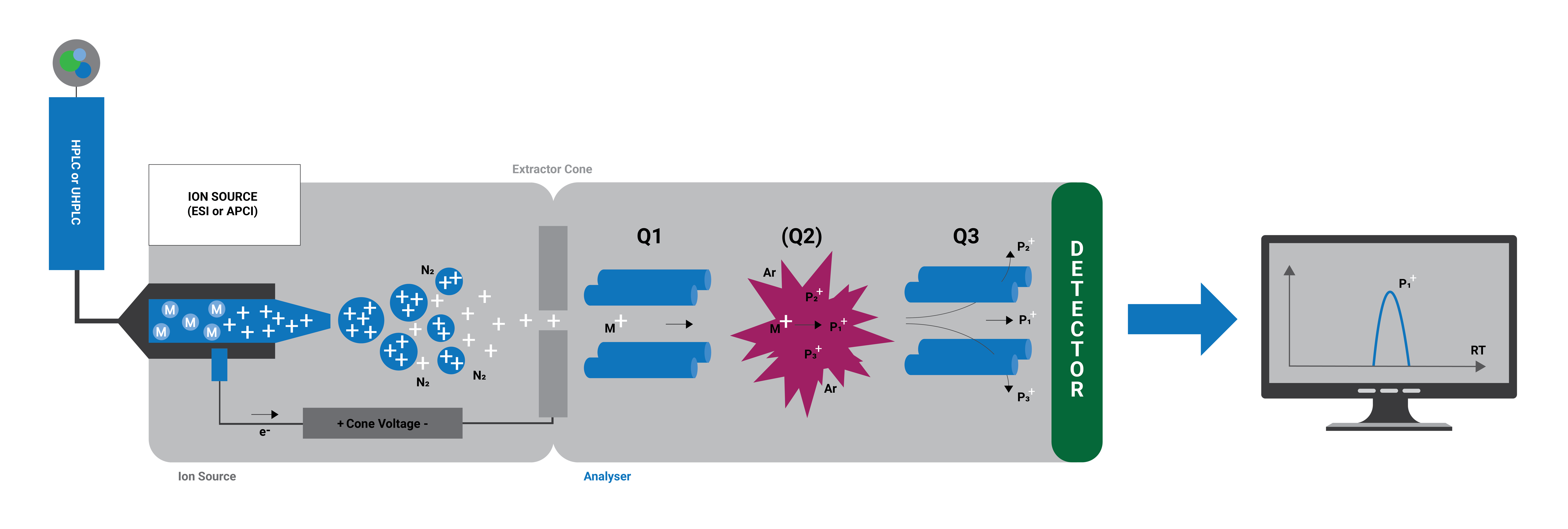
The 5 steps of LC/MS/MS
- After preparation, samples are separated and concentrated in a liquid chromatography instrument (HPLC).
- In the mass spectrometer’s ion source, molecules become charged ions. Different techniques for ionization have been developed based on the type of samples the MS will detect. The most common are:
- Electrospray ionization(ESI) produces ions using an electrospray in which a high voltage is applied to a liquid to create an aerosol.3 ESI, also known as the “soft ionization” technique, creates little fragmentation. For this reason, LC/MS/MS systems with ESI as an ionization technique are especially useful in producing ions from macromolecules because it overcomes the propensity of these molecules to fragment when ionized.4 The electrospray ionization technique was first reported by Masamichi Yamashita and John Fenn in 1984, who in 2002 was then awarded the Nobel Prize in Chemistry for the development of electrospray ionization for the analysis of biological macromolecules. 5 The soft ionizing nature of electrospray lends itself well to quantitative studies. As target molecules remain intact, a single ion is available for selection, allowing the maximum sensitivity of that analyte to be observed.
- Atmospheric Pressure Chemical Ionization (APCI) Source: As the polarity of a molecule decrease, so does electrosprays propensity to ionize them. It is for this reason APCI has been developed. The liquid is pumped through a capillary and nebulized at the tip. A corona discharge takes place near the tip of the capillary, initially ionizing gas and solvent molecules present in the ion source.
- In the mass spectrometer, the quadrupoles filter separates ions based on their mass-to-charge ratios (m/z). Within the triple quadrupole configuration, the first quadrupole isolates analyte molecules, the second quadrupole fragments select molecular ions, and the third quadrupole selectively isolates the ions for measurement via a detector.
- Ions and ion fragments reach the electrostatic detector and create a current that dedicated software translates into a mass spectrum.
- The mass spectrum plots the different mass-to-charge ratios (m/z) against their abundances (occurrence of a specific ion divided by the occurrence of the most plentiful ion) within the sample.
By providing highly sensitive and specific analysis of complex samples, LC/MS/MS has opened up new avenues of research. See how the PerkinElmer triple quadrupole LC/MS/MS portfolio can help labs achieve enhanced selectivity and sensitivity for the analyses of complex matrices.
REFERENCES
1. Yost, R. A.; Enke, C. G. (1978), “Selected ion fragmentation with a tandem quadrupole mass spectrometer” (PDF), Journal of the American Chemical Society, 100 (7): 2274, doi:10.1021/ja00475a072
2. https://en.wikipedia.org/wiki/Electrospray_ionization
3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2643089/
4. https://www.nobelprize.org/prizes/chemistry/2002/summary/

